Effect of chevron angle on heat transfer performance in plate heat exchanger using lithium bromide solution
Abstract
The objectives of this study are to investigate the effect of chevron angle on heat transfer performance in plate heat exchanger using lithium bromide solution and develop an empirical correlation for Nusselt number prediction. Plate heat exchangers with chevron angle of of 60° and 120° are tested in the present experiment. By varying the operational conditions, the heat transfer performance is evaluated. The results reveal that heat transfer rate, overall heat transfer coefficient and Nusselt number show 9%, 21% and 20% greater in plate heat exchanger with 120° chevron angle, respectively. And a correlation for predicting Nusselt number is developed with error less than 10%.
Keywords:
Absorption System, Plate Heat Exchanger, Chevron Angle, Nusselt Number1. Introduction
Heating and cooling devices have been widely adopted in industrial and resident applications. And these applications bring huge amount of energy consumption. What’s more, huge low grade heat is rejected to the surroundings as waste, which results in huge waste of energy and leads to environmental destruction. Absorption system has been proved to be a high efficient heat recovery technology since its appearance of about 1950s. Besides, absorption systems can benefit the environment by reducing the emission of global warming gas such as carbon dioxide.
Due to the active application of lithium bromide absorption system, many literatures concerning this system have been done both theoretically and experimentally. Omer and Muhsin1) investigated the effect of operating conditions on performance of absorption refrigeration system. And they concluded that the COP (coefficient of performance) value increases with increasing generator and evaporator temperature but decreases with increasing condenser and absorber temperatures. Okada et al.2) investigated the effect of concentration of lithium bromide solution on the COP. Papaefthimiou et al.3) proposed a mathematical model for analyzing the heat and mass transfer process of LiBr absorption on a horizontal tube. Besides, as a novel application of LiBr absorption system, solar-driven LiBr absorption system is also attracting the attention of researchers.4-10)
To improve the efficiency of absorption system, much effort have been done by using different refrigerants or changing the structure of the heat exchangers.11,12) For the existing LiBr absorption cycle, Shell and tube heat exchanger and plate heat exchanger are most commonly used as solution heat exchanger. The heat transfer efficiency of the solution heat exchanger impact the system performance a lot. Compared to the shell and tube heat exchanger, the COP of the system can be improved up to 12% by using plate heat exchanger. And for the large scale absorption systems that produced in the domestic market, shell and tube heat exchanger is normally selected13). However, plate heat exchangers are used as solution heat exchanger in many developed countries even in small-scale absorption system.
Since little literature related to heat transfer performance of LiBr solution in plate heat exchanger can be found, one of the objectives of this study is to investigate the effect of chevron angle on heat transfer performance in plate heat exchanger using lithium bromide solution. Besides, the existing empirical correlations of Nusselt number are almost derived from experiments that using water as working fluid. In this study, an empirical correlation for Nusselt number prediction would be developed based on the experimental results of LiBr solution.
2. Experimental set-up and method
2.1 Experimental set-up and operational conditions
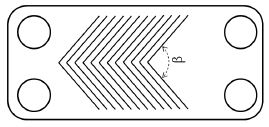
In this experiment, plate heat exchangers with chevron angle of 60° and 120° are tested. The schematic of the tested solution heat exchanger is shown in Fig. 1. And the specification is listed in Table 1. The heat exchanger that is consisted of 24 plates and 12 channels in both cold and hot side.
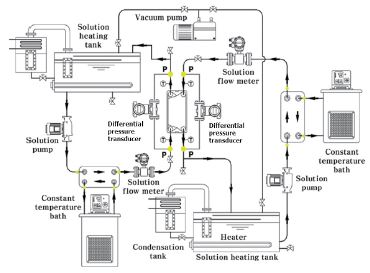
Fig. 2 shows the experimental set-up. The tube and all the components are well insulated to prevent heat loss. Two 15 kW heaters are installed in two solution heating tanks respectively to keep the solution temperature at constant. The concentrations of LiBr solution at high temperature and low temperature sides are 62.5% and 58.5%, respectively. Mass flow rates at inlet for both sides are measured by two solution flow meters (Toshiba, LF430DL), respectively. And the temperature is measured through thermocouple (Omega, T-type). And the pressure differences between inlet and outlet of the solution heat exchanger for both sides are measured through differential pressure transducers (Rosemount, 3051S), respectively.
All the data are obtained through a data recorder after calibration. The uncertainties of the mass flow meter, temperature and pressure are ±0.1%, ±0.15% and ±0.1%, respectively.
The operational conditions are listed in Table 2. The temperature of cold side is kept constant at 7 0℃, and the hot side temperature is 155℃. The mass flow rate at cold side is kept at 380 kg/h, and hot side is varying from 150 kg/h to 550 kg/h.
2.2 Experimental method
Heat transfer rate for cold and hot side can be calculated as shown in Eq. (1) and Eq. (2).
| (1) |
| (2) |
The overall heat transfer coefficient is also a very important parameter to evaluate performance of heat exchanger. It can be calculated as shown in Eq. (3).
| (3) |
Where △TLMTD and Qave are logarithmic mean temperature and average mass flow rate, respectively. They can be calculated according to Eq. (4) and Eq. (5).
| (4) |
| (5) |
Some important dimensionless numbers that used in evaluating heat transfer performance are also calculated. Reynolds number for hot and cold side in the solution heat exchanger can be calculated as :
| (6) |
Prandtl number and Nusselt number can be calculated according to Eq. (7) and Eq. (8).
| (7) |
| (8) |
Heat transfer coefficient in Eq. (8) can be calculated from Eq. (9)
| (9) |
3. Experimental results and discussion
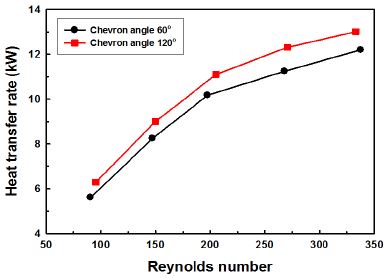
Fig. 3 shows the variation of heat transfer rate with Re number. The heat transfer rate increases with increasing Reynolds number. And the heat transfer rate of 120° chevron angle is about 9% higher than that of 60° chevron angle in average. The reason is that the larger chevron angle leads to more complex flow motion in the plate heat exchanger, this results in the heat transfer enhancement.
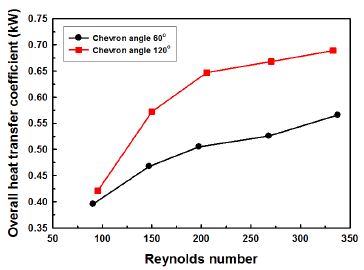
Fig. 4 shows the variation of overall heat transfer coefficient with Re number. The overall heat transfer coefficient increases with increasing Reynolds number. And the overall heat transfer coefficient of 120° chevron angle is about 21% higher than that of 60° chevron angle in average. The impact of chevron angle on overall heat transfer coefficient tends to become greater with the increasing Reynolds number.
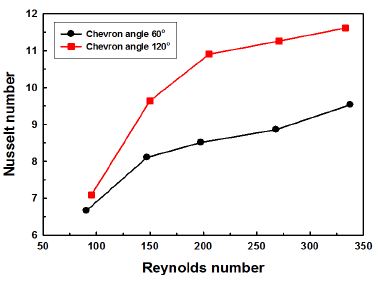
A similar tendency can be observed in Fig. 5. The Nusselt number increases with increasing Reynolds number. And the Nusselt number of 120° chevron angle is about 20% higher than that of 60° chevron angle in average. The impact of chevron angle on Nusselt number tends to become greater with increasing Reynolds number.
| (10) |
| (11) |
| (12) |
Empirical correlations are widely adopted to analyze the heat transfer performance of plate heat exchanger, among them, Eqs. (10-12) are the most frequently used ones.14-16)
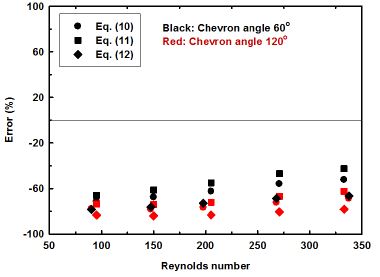
Nusselt number of the present experiment is also calculated by using this correlation, and the results are shown in Fig. 6. As shown in this figure, all of the predicted data deviate from the experimental ones over 40%. It means that the existing correlations are not applicable for the LiBr solution. Due to this reason, a new correlation is derived according to the experimental results as shown below:
| (13) |
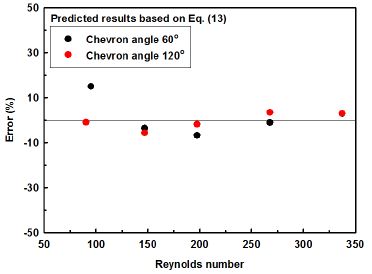
The data predicted through the new derived Eq. (13) are shown in Fig. 7. 90% of the predicted results deviate from experimental ones within 10%, the average deviation is 3.3%. The predicted results well matched with the experimental ones.
4. Conclusions
This study investigated the impact of chevron angle of plate heat exchanger on heat transfer performance of LiBr solution. As a result, heat transfer rate, overall heat transfer coefficient and Nusselt number show 9%, 21% and 20% greater value in plate heat exchanger with 120° chevron angle, respectively. Since the existing correlations for predicting Nusselt number are not applicable for the LiBr solution, a new correlation is developed based on the experimental results, and 90% of the predicted results agree well with the experimental ones with error less than 10%.
― Nomenclature ―
| Cp : | Specific heat [J/kg·K] |
| Dh : | Hydraulic diameter [m] |
| h : | Heat transfer coefficient [W/m2·K] |
| k : | Conductivity [W/m·k] |
| : | Mass flow rate [kg/h] |
| Nu : | Nusselt number |
| Pr : | Prandtl number |
| Re : | Reynolds number |
| Q : | Heat transfer rate [W] |
| T : | Temperature [℃] |
| U : | Overall heat transfer coefficient [W/m2·℃] |
| v : | Velocity [m/s] |
Greek Symbols
| β : | Chevron angle |
| ρ : | Density [kg/m3] |
| μ : | Viscosity [kg/m·s] |
Subscripts
| c : | Cold side |
| h : | Hot side |
| i : | Inlet |
| ave : | Average |
| o : | Outside |
| m : | metal |
Acknowledgments
This work was supported by the Korea Institute of Energy Technology Evaluation and Planning(KETEP) and the Ministry of Trade, Industry & Energy(MOTIE) of the Republic of Korea (No. 20152020001240).
References
-
O. Kaynakli, and M. Kilic, (2007), "Theoretical study on the effect of operating conditions on performance of absorption refrigeration system", Energy Conversion and Management, Vol. 48, p599-607.
[https://doi.org/10.1016/j.enconman.2006.06.005]
- K. Okada, M. Ono, and T. Tomimura, (1972), "Design and heat transfer characteristics of a new plate heat exchanger", Heat Transfer, Vol. 1(No. 1), p90-95.
-
V. D. Papaefthimious, D. C. Karampions, and E. D. Rogdakis, (2006), "A detailed analysis of water-vapor absorption in LiBr-H2O solution on acooled horizontal tube", Applied Thermal Engineering, Vol. 26, p2095-2102.
[https://doi.org/10.1016/j.applthermaleng.2006.04.010]
-
N. I. Ibrahim, F. A. Al-Sulaiman, and F. N. Ani, (2017), "Performance characteristics of a solar driven lithium bromide-water absorption chiller integrated with absorption energy storage", Energy Conversion and Management, Vol. 150, p188-200.
[https://doi.org/10.1016/j.enconman.2017.08.015]
-
E. D. Kerme, A. Chafidz, O. P. Agboola, J. Orfi, A. H. Fakeeha, and A. S. Al-Fatesh, (2017), "Energetic and exergetic analysis of solar-powered lithium bromide-water absorption cooling system", Journal of Cleaner Production, Vol. 151, p60-73.
[https://doi.org/10.1016/j.jclepro.2017.03.060]
-
M. Li, C. Xu, R. H. E. Hassanien, Y. Xu, and B. Zhuang, (2016), "Experimental investigation on the performance of a solar powered lithium bromide-water absorption cooling system", International Journal of Refrigeration, Vol. 71, p46-59.
[https://doi.org/10.1016/j.ijrefrig.2016.07.023]
- G. Yaxiu, W. Yuyuan, and K. Xin, (2008), "Experimental research on a new solar pump-free lithium bromide absorption refrigeration system with a second generator", Solar Energy, Vol. 82(No. 1), p33-42.
-
M. Mazloumi, M. Naghashzadegan, and K. Javaherdeh, (2008), "Simulation of solar lithium bromide-water absorption cooling system with parabolic trough collector", Energy Conversion and Management, Vol. 49(No. 10), p2820-2832.
[https://doi.org/10.1016/j.enconman.2008.03.014]
-
A. Iranmanesh, and M. A. Mechrabian, (2014), "Optimization of a lithium bromide-water solar absorption cooling system with evacuated tube collectors using the genetic algorithm", Energy and Buildings, Vol. 85, p427-435.
[https://doi.org/10.1016/j.enbuild.2014.09.047]
-
A. A. Heidari, and K. Shiati, (2005), "Using the novel technology of desailinating seawater by solar cell & lithium bromide absorption chiller in rural area", Desalination, Vol. 183(No. 1-3), p23-27.
[https://doi.org/10.1016/j.desal.2005.02.042]
- C. K. Park, O. K. Kwon, C. G. Moon, and J. I. Yoon, (1998), "Characteristic Analysis of Single Effect Absorption using New Working Solution", Journal of the Korea Society For Power System Engineering, p71-77.
- K. T. Lee, H. S. Lee, C. G. Moon, K. C. Kang, and J. I. Yoon, (2004), "Experimental Study on Performance Characteristics of Absorber with Variations of Tube Diameters", Journal of the Korea Society For Power System Engineering, p328-333.
-
O. K. Kwon, D. A. Cha, J. H. Yun, and H. S. Kim, (2009), "Performance evaluation of plate heat exchanger with chevron angle variations", Journal of Mechanical Science and Technology (B), Vol. 33(No. 7), p520-526.
[https://doi.org/10.3795/KSME-B.2009.33.7.520]
- K. Sdic, (2002), "Heat exchangers: Selection, Rating, and Thermal Design", second edition, CRC Press, Florida, USA.
-
J. Soontarapiromsook, O. Mahian, A. S. Dalkilic, and S. Wongwises, (2018), "Effect of surface roughness on the condensation of R134a in vertical chevron gasketed plate heat exchangers", Experimental Thermal and Fluid Science, Vol. 91, p54-63.
[https://doi.org/10.1016/j.expthermflusci.2017.09.015]
-
K. Nilpueng, T. Keawkamrop, H. S. Ahn, and S. Wongwises, (2018), "Effect of chevron angl and surface roughness on thermal performance of single-phase water flow inside a plate heat exchangers", International Communications in Heat and Mass Transfer, Vol. 91, p201-209.
[https://doi.org/10.1016/j.icheatmasstransfer.2017.12.009]